O F Cl S concluding that O F and Cl have smaller atomic radius than S. It must be noted atoms lack a well-defined outer boundary.

3 2 Trends In Size Problems Chemistry Libretexts
1 Count the electrons all of them in each atom or ion.

. Yes and yes but the reasoning is no very good. This will compress the energy levels a bit and make the ionic radius smaller for the potassium cation. Sulphur S has the largest atomic radius beaches on moving left to right in a period Atomic Radius decreases.
2 What happens to the orbitals and thus to atoms or ions with a given number of electrons let us say 18 electrons when you increase the atomic number of the element more protons in the nucleus. So far you can rank the atomic radius of sulfur chlorine fluorine and oxygen in increasing order as. In general both have the same electrons repulsing each other the same amount but the Cl Chlorine has more protons which would attract more.
Sulfur and oxygen are both in column 16 of a wide form periodic table with sulfur below oxygen. O2 is larger than O because O is neutral but O2 has a charge 2. Therefore the chloride anion will have the larger atomic radius.
Show activity on this post. The negative ions are larger than their neutral atom because the electrons all going into the outer shell which is already occupied with electrons repel each other and that takes up more space. Think of it this way.
The atomic radius of a chemical element is a measure of the distance out to which the. So the general answer is the Cl will become a tighter configuration in 3D. O2 is larger than O because the increase in electron repulsions that accompany addition of an electron causes the electron cloud to expand.
O2 is larger than O because the increase in electron repulsions that accompany addition of an electron causes the electron cloud to expand. Answer 1 of 2. The atomic radius of Sulfur atom is 105pm covalent radius.
Atomic radius is a trend from the periodic table. Sulfur and oxygen are in the same group 16 with sulfur directlly below oxygen so sulfur the atomic radius of sulfur is larger than the atocmi radius of oxygen. In other words K has bigger effective nuclear charge than Cl which translates to a bigger net positive charge felt by the outermost electrons.
Also SH- is a much weaker base than OH. Is O or O2 larger. Which has a larger radius S or CL.
S is more polarizable. Atomic radius is smallest at the far right of the periodic table O and F and increases as you move left towards Be and Li. Is O or O2 larger.
Atomic Radius of Sulfur. SulphurS has the largest atomic radius beaches on moving left to right in a period Atomic Radius decreases. Does S have a larger atomic radius than O.
Atomic radius also increases as you move down the periodic table from F to At for example. Because S has a larger atomic radius than O the conjugate base of hydrogen sulphide is more stable than the conjugate base of water. Which ion has the larger atomic radius.
O2 is larger than O because O is neutral but O2 has a charge 2.
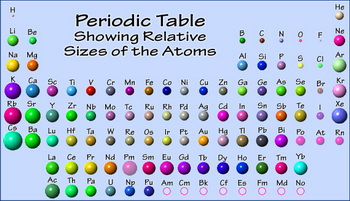
What Is The Trend In Atomic Radius From Left To Right On The Periodic Table Socratic
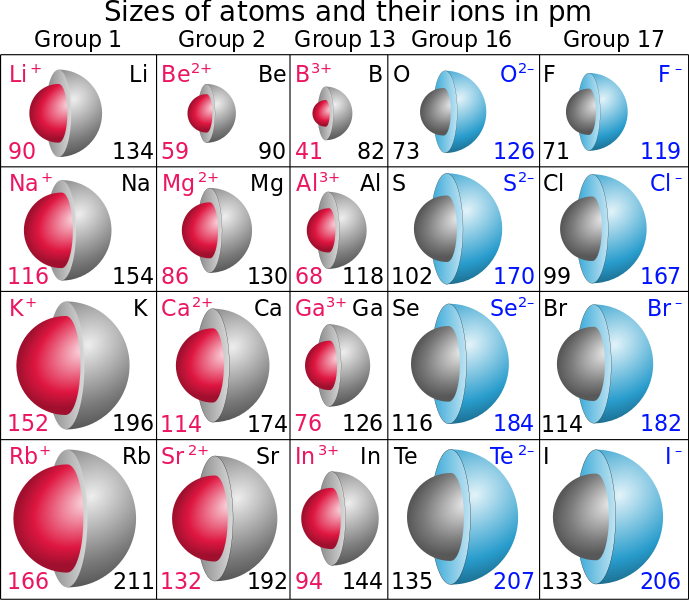
Which Of The Entities Have Higher Atomic Radius A Ca Ca2 B S S2 Please Explain It Socratic


0 Comments